CUREXO Gains CE Mark Approval for CUVIS-spine Robot
Date : 2020-08-12
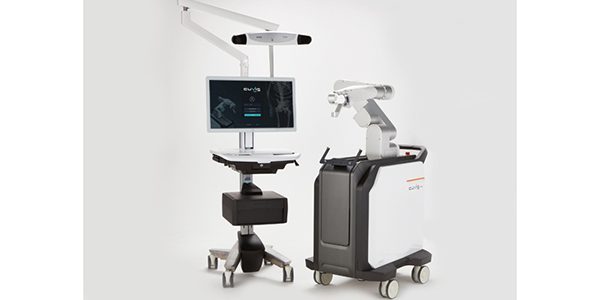
Curexo received approval under the CE Mark for the CUVIS-spine surgical robot. The company is also planning to apply for FDA clearance.
CUVIS-spine guides and supports the pedicle screw to the correct position using a robotic arm, one-step surgical tools and a real-time location sensor. Real-time patient position monitoring can correct the surgical plan in real time, and can help reduce radiation exposure for the patient and O.R. staff.
CUVIS-spine provides imaging compatibility on not only C-arms, but also O-arms. The device received approval by Korea's Ministry of Food and Drug Safety and launched late last year in the region.
The company is also developing CUVIS-joint for potential launch in 2020.
According to the CEO of CUREXO, "Certifying the CE certificate for CUVIS-spine is another big step taken after last year's MFDS certificate. Also, achieving the CE certificate for the CUVIS-spine is meaningful because now CUREXO is aiming for the global market with a self-developed surgical robot. The following task will be FDA approval and entering the U.S. market, which is number one in surgical robots.
https://www.orthoworld.com/curexo-gains-ce-mark-approval-for-cuvis-spine-robot/





